Home /
Expert Answers /
Chemical Engineering /
the-liquid-phase-irreversible-reaction-a-gt-b-c-is-carried-out-in-a-cstr-to-learn-the-rate-law-the-pa935
(Solved): The liquid phase irreversible reaction A->B+C is carried out in a CSTR. To learn the rate law the ...
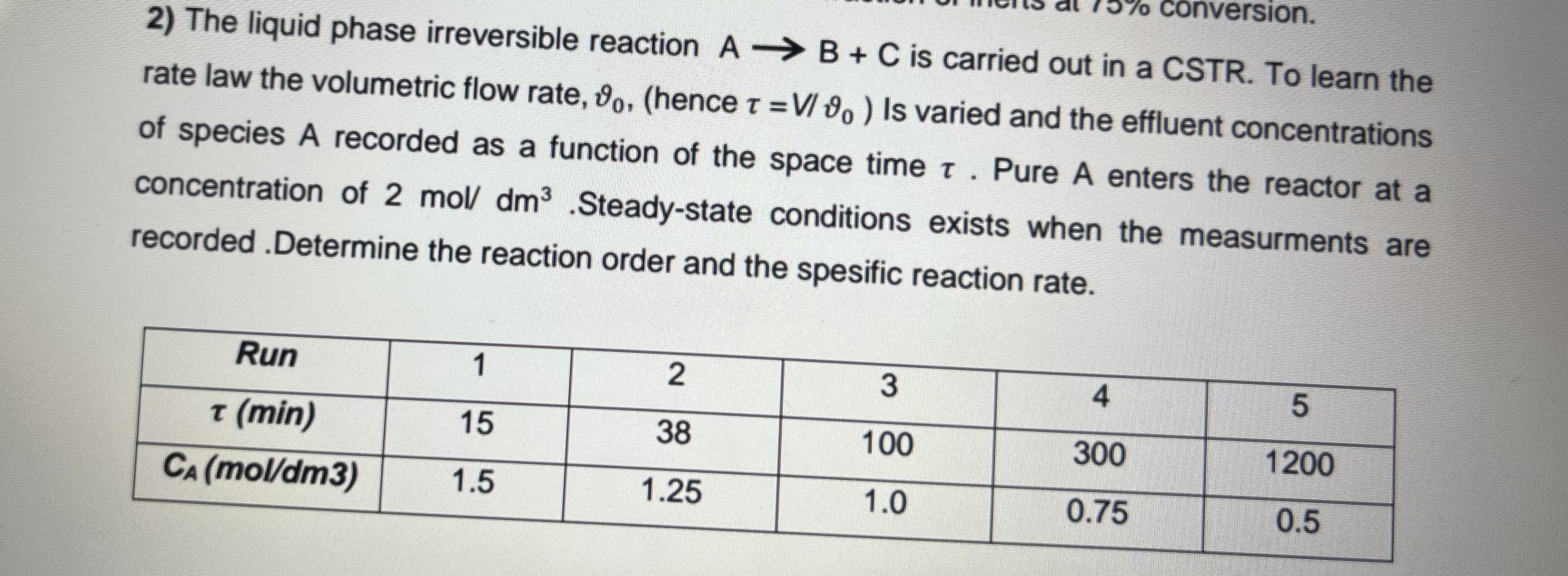
The liquid phase irreversible reaction A->B+C is carried out in a CSTR. To learn the rate law the volumetric flow rate, var\theta _(0)\tau =(V)/(v)ar\theta _(0) A recorded as a function of the space time \tau . Pure A enters the reactor at a concentration of 2mo(l)/(d)m^(3).Steady-state conditions exists when the measurments are recorded .Determine the reaction order and the spesific reaction rate.
\table[[Run,1],[2,3],[4,5],[\tau (min),15],[38,100],[300,1200],[C_(A) (mo(l)/(d)m3),1.5],[1.25,1.0],[0.75,0.5]]