Home /
Expert Answers /
Chemistry /
question-completion-status-moving-to-another-question-will-save-this-response-qustion-19-matc-pa327
(Solved): . Question Completion Status: Moving to another question will save this response. Qustion 19 Matc ...
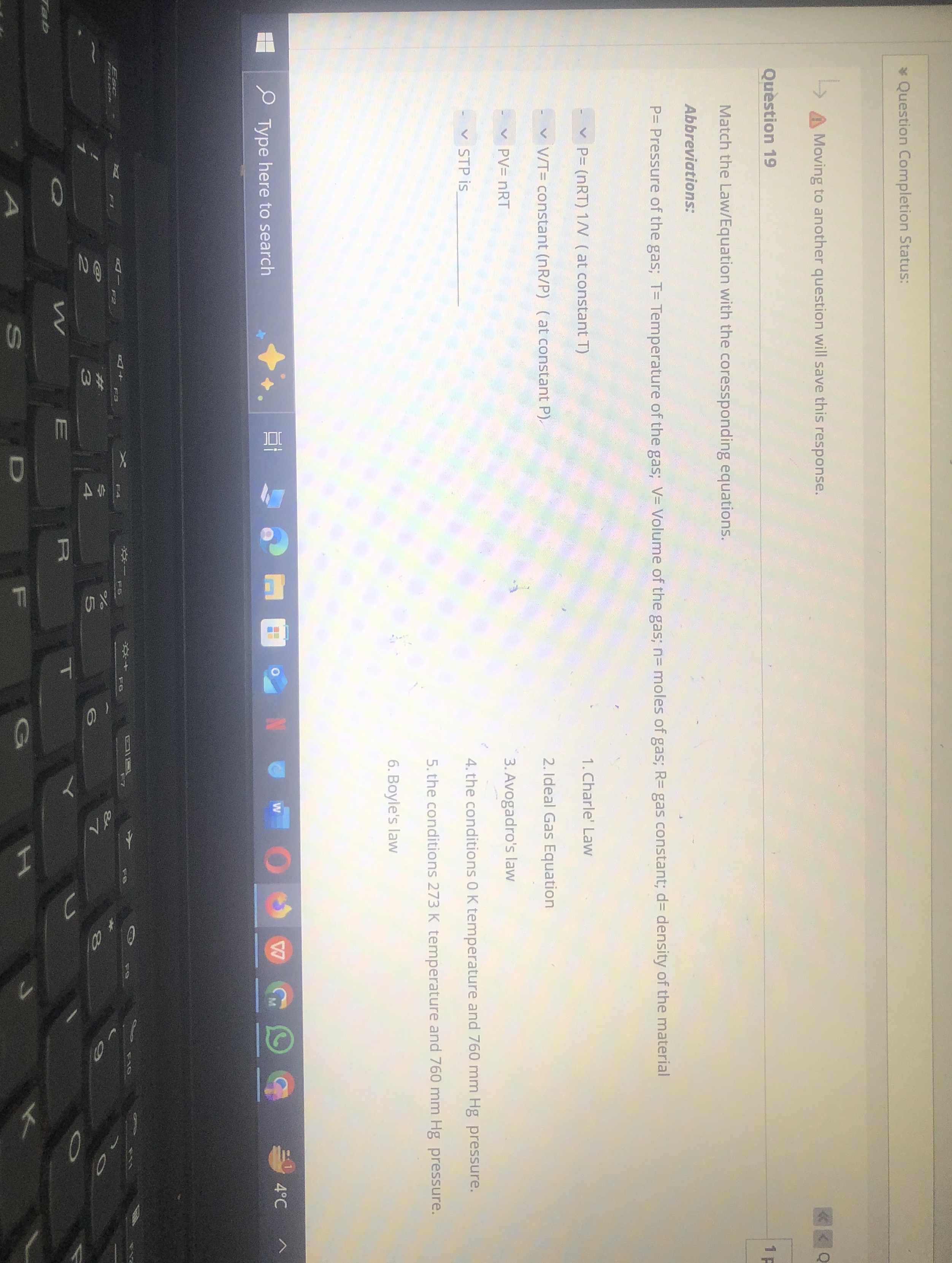
.Question Completion Status: Moving to another question will save this response. Quèstion 19 Match the Law/Equation with the coressponding equations. Abbreviations:
P=Pressure of the gas;
T=Temperature of the gas;
V=Volume of the gas;
n=moles of gas;
R=gas constant;
d=densityof the material
P=(nRT)1N(at constant
TCharle' Law
(V)/(T)=constant (
n(R)/(P)) (at constant P ). Ideal Gas Equation
PV=nRT
◻STP is
q,Avogadro's law the conditions 0 K temperature and 760 mm Hg pressure. the conditions 273 K temperature and 760 mm Hg pressure. Boyle's law