Home /
Expert Answers /
Physics /
an-ideal-gas-at-16-5-deg-c-and-a-pressure-of-2-28-times-10-5-pa-occupies-a-volume-of-2-77m-3-a-pa562
(Solved): An ideal gas at 16.5\deg C and a pressure of 2.28\times 10^(5)Pa occupies a volume of 2.77m^(3). (a) ...
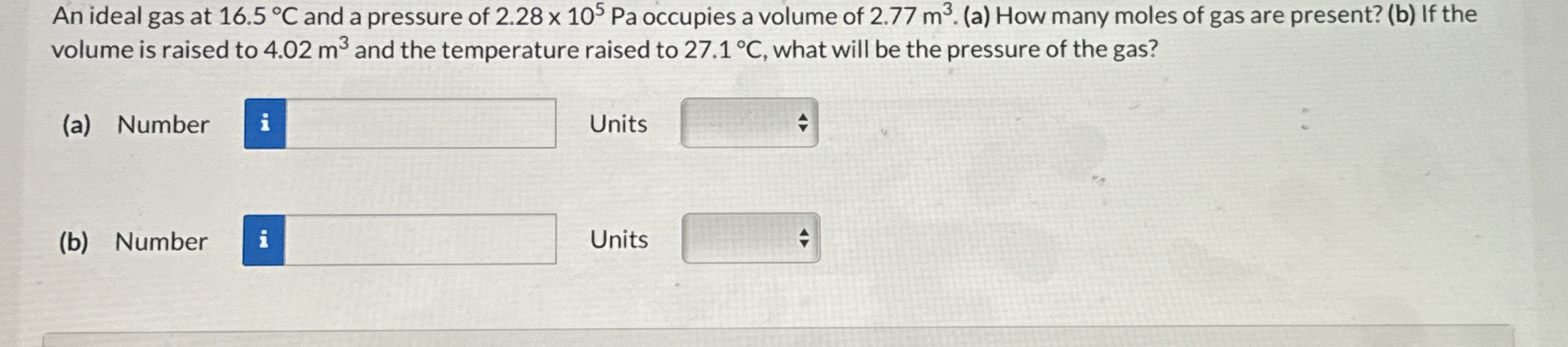
An ideal gas at
16.5\deg Cand a pressure of
2.28\times 10^(5)Paoccupies a volume of
2.77m^(3). (a) How many moles of gas are present? (b) If the volume is raised to
4.02m^(3)and the temperature raised to
27.1\deg C, what will be the pressure of the gas? (a) Number
◻Units
◻(b) Number
◻Units