Home /
Expert Answers /
Chemistry /
a-particular-chemical-reaction-has-delta-h-deg-400kjmol-1-and-delta-s-deg-80jk-1-mol-1-pa269
(Solved): A particular chemical reaction has \Delta H\deg =-400kJmol^(-1) and \Delta S\deg =-80JK^(-1)mol^(-1) ...
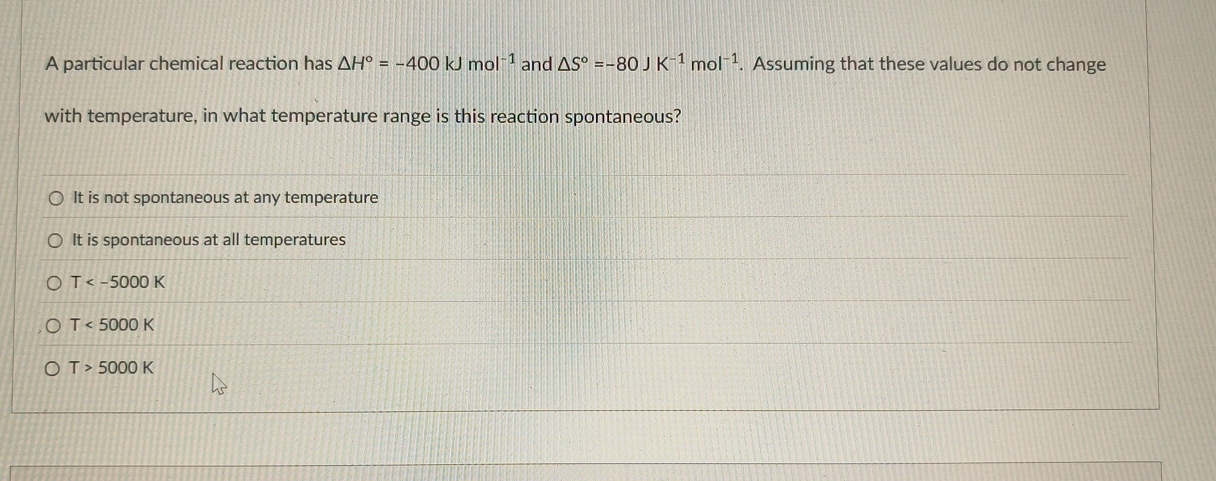
A particular chemical reaction has
\Delta H\deg =-400kJmol^(-1)and
\Delta S\deg =-80JK^(-1)mol^(-1). Assuming that these values do not change with temperature, in what temperature range is this reaction spontaneous? It is not spontaneous at any temperature It is spontaneous at all temperatures
T<-5000K
T<5000K
T>5000K