Home /
Expert Answers /
Chemistry /
a-galvanic-cell-zn-zn-2-ni-2-ni-runs-spontaneously-if-a-current-is-imposed-to-turn-this-int-pa259
(Solved): A galvanic cell Zn|Zn^(2+)||Ni^(2+)| Ni runs spontaneously. If a current is imposed to turn this int ...
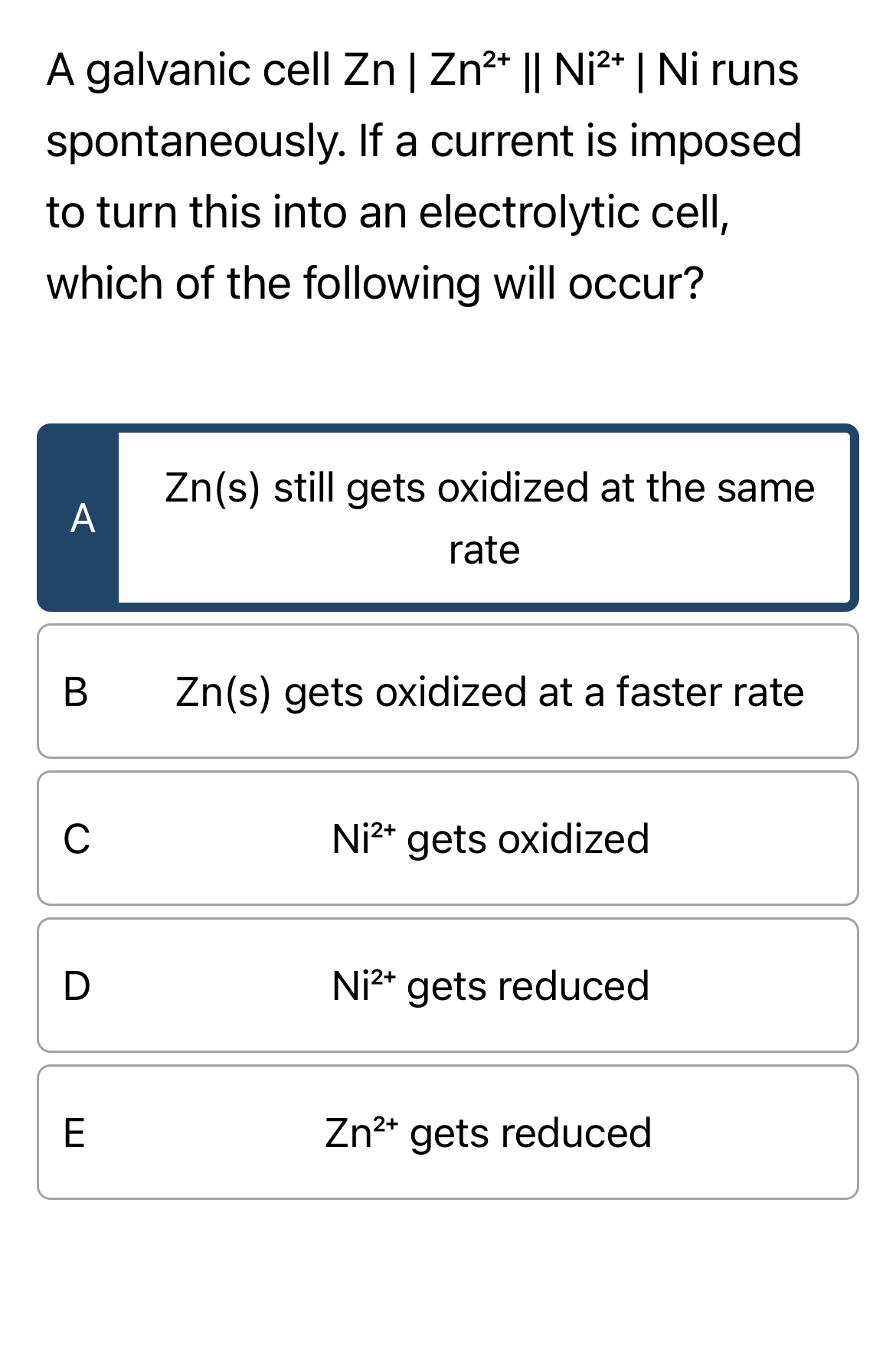
A galvanic cell
Zn|Zn^(2+)||Ni^(2+)|Ni runs spontaneously. If a current is imposed to turn this into an electrolytic cell, which of the following will occur?
Zn(s)still gets oxidized at the same rate B
,Zn(s)gets oxidized at a faster rate C
,Ni^(2+)gets oxidized D
,Ni^(2+)gets reduced
E,Zn^(2+)gets reduced