Home /
Expert Answers /
Chemistry /
a-chemist-measures-the-enthalpy-change-delta-h-during-the-following-reaction-2ch-3-oh-i-h-2-so-pa737
(Solved): A chemist measures the enthalpy change \Delta H during the following reaction: 2CH_(3)OH(I)+H_(2)SO_ ...
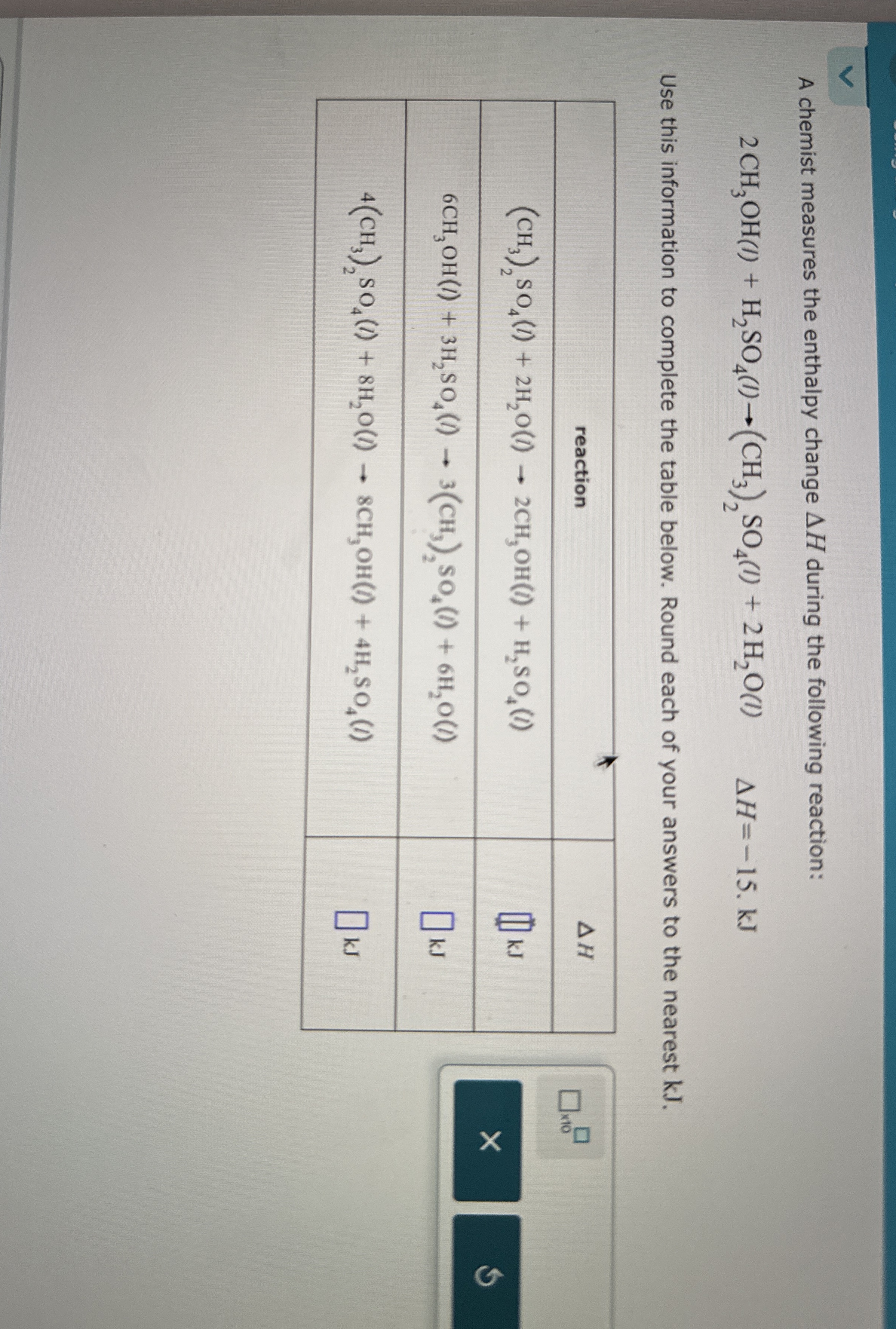
A chemist measures the enthalpy change
\Delta Hduring the following reaction:
2CH_(3)OH(I)+H_(2)SO_(4)(I)->(CH_(3))_(2)SO_(4)(I)+2H_(2)O(l),\Delta H=-15.kJUse this information to complete the table below. Round each of your answers to the nearest kJ. \table[[reaction,
\Delta H