Home /
Expert Answers /
Chemistry /
4-39-b-determine-the-equilibrium-constant-of-the-following-reaction-conducted-at-330-deg-c-10-a-pa714
(Solved): 4^(') (b) Determine the equilibrium constant of the following reaction conducted at 330\deg C [10] a ...
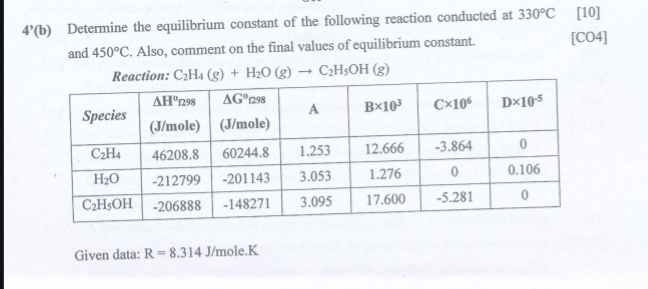
4^(')(b) Determine the equilibrium constant of the following reaction conducted at
330\deg C[10] and
450\deg C. Also, comment on the final values of equilibrium constant. [CO4] Reaction:
C_(2)H_(4)(g)+H_(2)O(g)->C_(2)H_(5)OH(g)\table[[Species,
\Delta H^(0)E298
((J)/( m)ole ),
\Delta G^(0)ng9
((J)/(m)ole),A,
B\times 10^(3),C
\times 10^(6),D
\times 10^(-5)